Keywords
Phase change, phase transition, fusion, solidification, freezing, vaporization, liquefaction, sublimation, condensation, enthalpy, thermodynamics, Pressure Temperature diagram, Food industry, non-stationary equilibrium
Context / Goal
Thermodynamic phase change is due to a local variation of pressure, volume or temperature. An energetic exchange occurs during this phase change, and is characterized by a modification in the molecular structure of the considered material.
Phase change processes are very useful in Industrial technologies. Some examples :
- Electromagnetic stirring : Fusion of a solid charge into liquid, then freezing
- Transport of products in solid state: solidification and condensation to minimize risk of leakage when transporting the container
- Food processing industry: to cook food, mainly evaporation
- Use of SMART materials to store energy.
For all of these applications, an accurate knowledge of local pressure and temperature fields is required to optimize energy transfer. Modeling these processes is therefore a good tool to understand the underlying physical phenomena, to quantify them and to increase process efficiency.
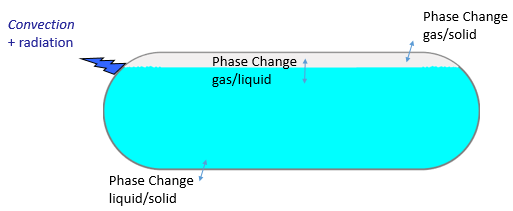
The proposed model studies the phase change Solid/Liquid/Gas due to some thermal exchanges between the container and its neighborhood. Initially, two fluid phases are present (gas and liquid). According to the thermodynamic diagram, a solid phase could appear through solidification or condensation.
For the purpose of confidentiality, the application of this model is kept secret, as is the name of the CLIENT.
SIMTEC's Achievements / Results
The first mission of SIMTEC was to visit the CLIENT on-site, to visualize industrial installation and to work on the specifications with the CLIENT. The retained numerical model enabled consideration of every phase change without assuming anything. These phase changes follow the thermodynamic diagram that the user has to specify as an input data.
In this model, the following physical phenomena are considered :
-
Heat transfer: conduction inside fluid and solid phases (an equivalent conductivity approach has been taken into consideration to decrease the complexity due to convection), surface to ambient radiation, cooling of the container by free convection. Energy exchanges due to phase change (positive or negative) are taken into consideration with the latent heat.
-
Multi-phasic Approach: Arbitrary Lagrangien Eulerian Approach (ALE) to distinguish the fluid phase from the solid one. Interface tracking method to make the distinction between the liquid phase and the gas phase. Thus, the level of liquid can vary according to time during the simulation.
This work has enabled us to develop a numerical model which has been validated by comparisons with experimental results. After this validation, it enables us to study the influence of the cooling system on the solidification kinetics and to optimize it.